About the lab
Epithelium and mesenchyme derivatives are key tissue types that govern morphogenesis and functioning of overwhelming majority of organs in our bodies. Cell-cell interactions between cells inside one tissue type and across tissue boundaries maintain proper functioning and regeneration of tissues and organs. Regeneration, both physiological and post-traumatic, is provided by multiplication of adult stem cells. In case of regeneration failure tissue engineering gives hope for restoration of organs and tissues lost.
Our laboratory focuses on projects in the field of epithelial tissue biology, epithelia-mesenchymal interactions, morphogenesis, stem cells, tissue engineering and regenerative medicine.
Research fields
Skin and hair follicles
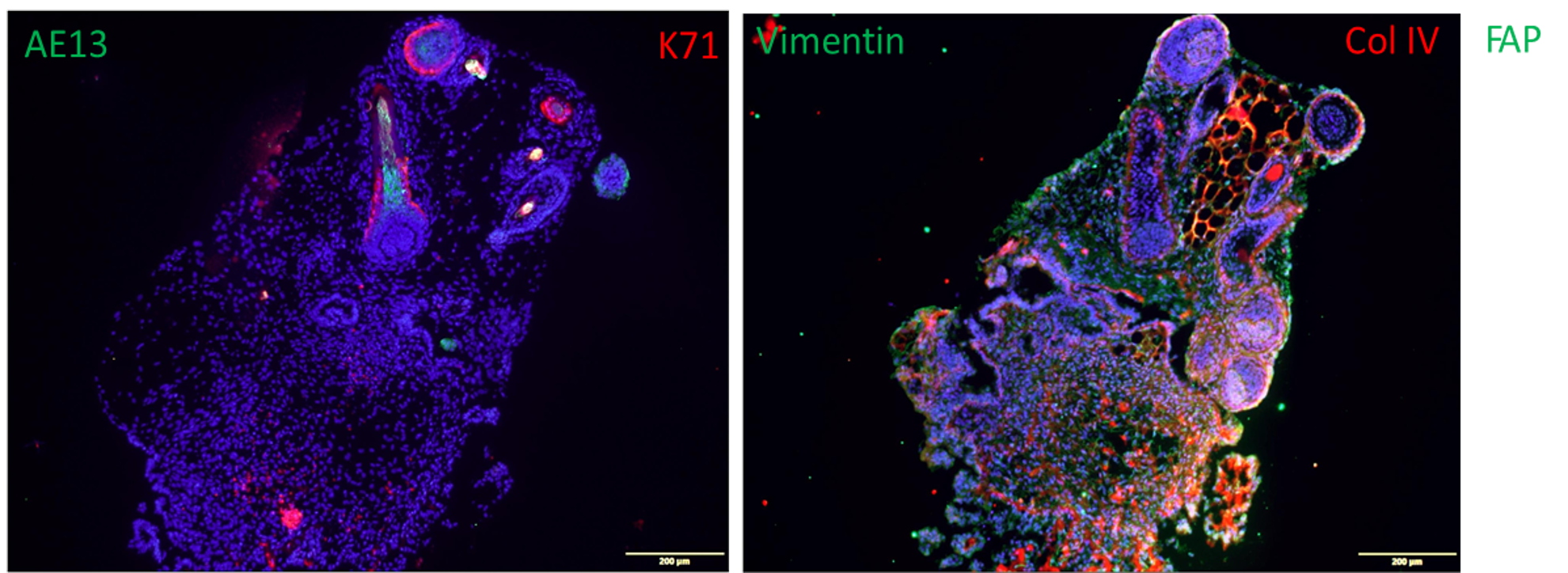
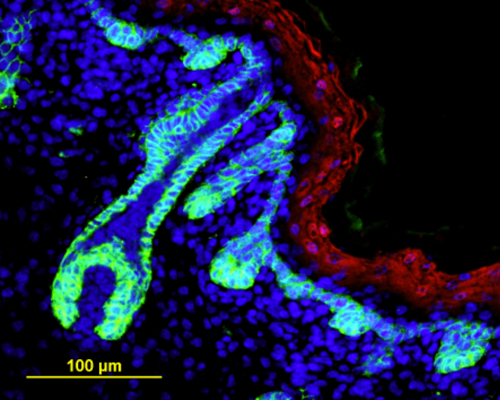
Fig. 1. Organoids made from human iPSCs (110 days) show the formation of hair follicles which express AE13 and Keratin 71 (K71). Also shown is the expression of vimentin and Collagen IV.
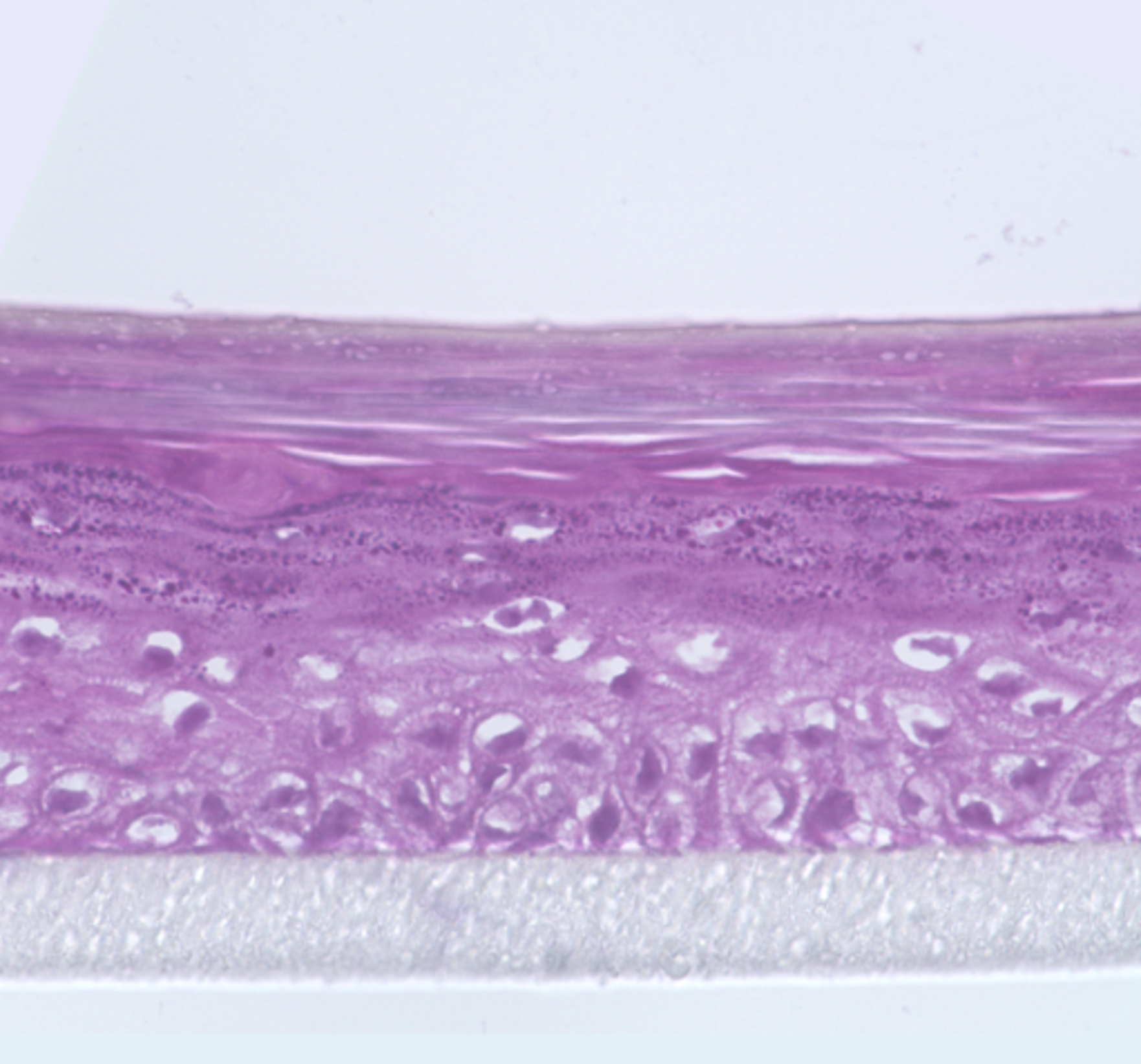
We are interested in developing novel approaches for healing tissue defects (for example, burns and skin wounds) with cultured human skin cells (fibroblasts and keratinocytes) and specially engineered constructs named tissue equivalents composed of cultured cells seeded on scaffolds to mimic the natural structure of tissues. Our lab was established for research in the field of skin biology. Therefore, we have developed several types of skin equivalents which have been implemented in medical practice. Gradually we have come to be interested in the biology and development of skin appendages, that is, skin derivatives developing and functioning as part of it, such as hair follicles and glands. We try to reconstruct hair follicles from adult and pluripotent stem cells in order to understand cell-cell interactions better, especially during hair follicle generation in embryogenesis and also to make progress in the treatment of severe alopecia (balding). Currently, we focus on the generation of fully functional skin equivalents with appendages, including those derived from pluripotent stem cells.
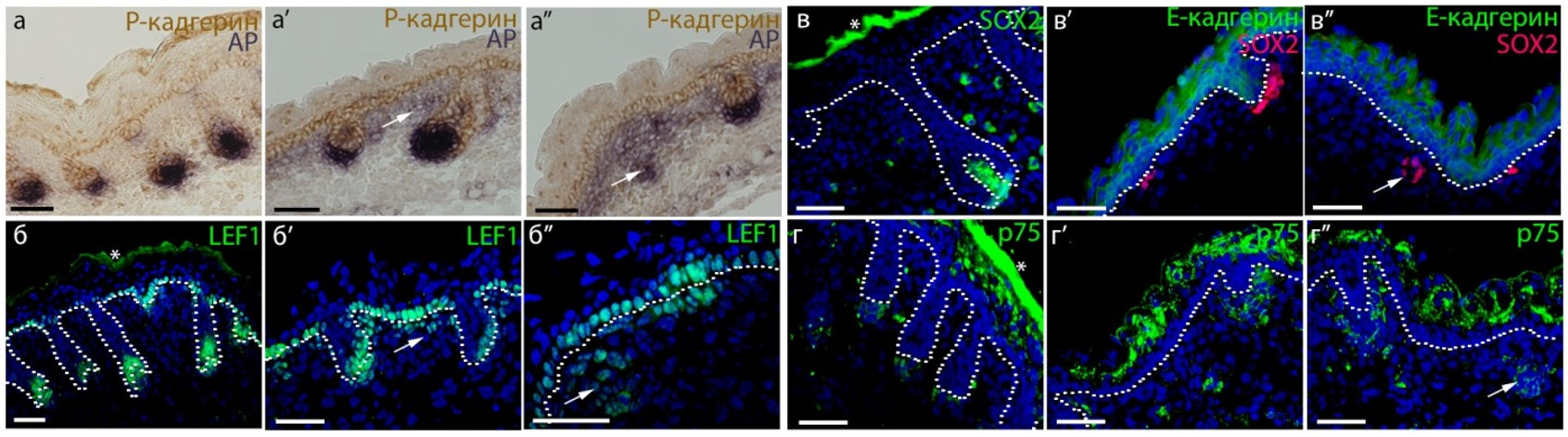
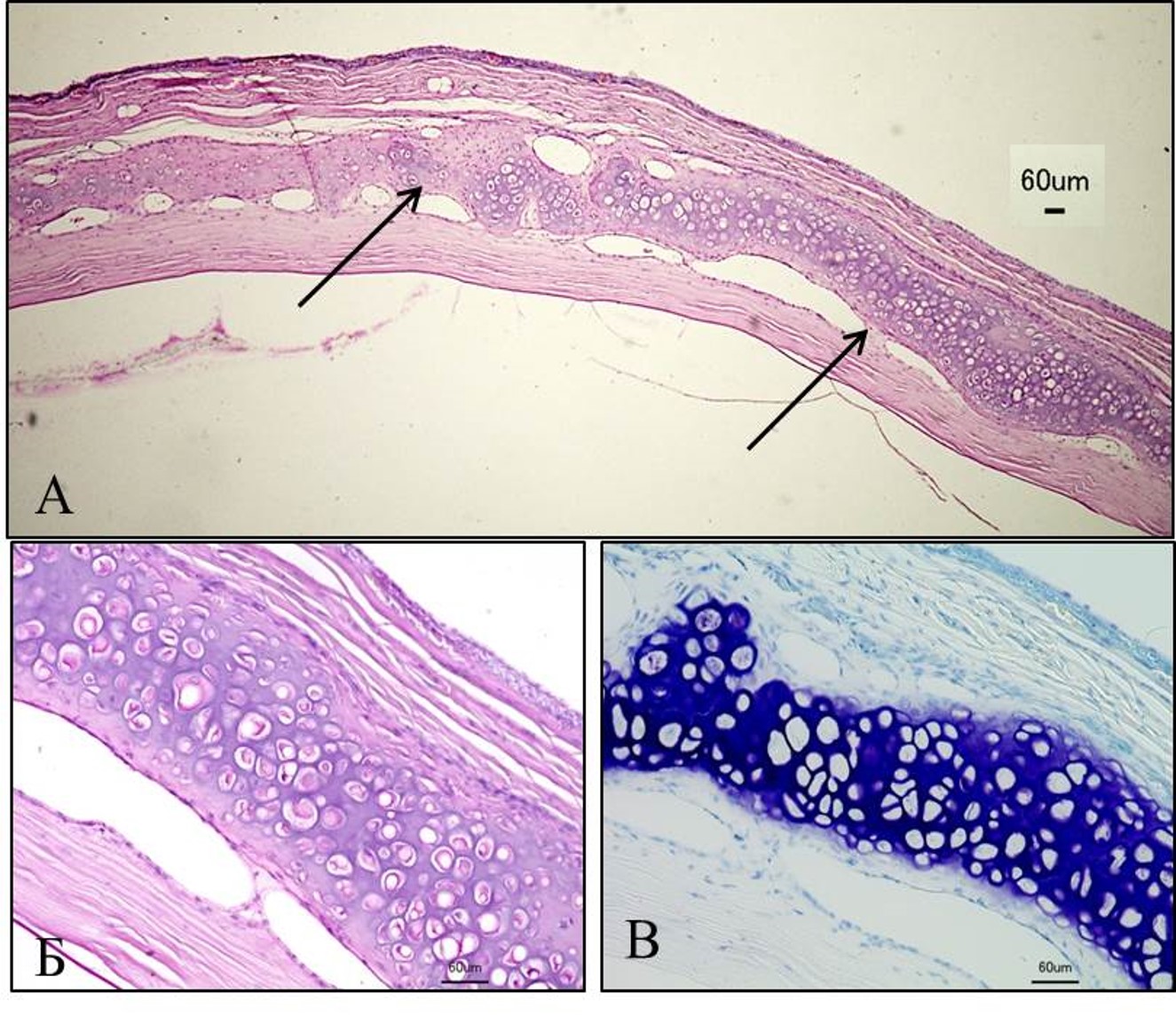
Fig. 2. Cross-section of embryonic (E18.5) we/we wal/wal mutant mouse skin and the expression of markers of the dermal component of hair follicle (Rippa et al., 2015).
Tissue engineering
We have many years of experience working with different types of human cells including stem and progenitor ones: skin fibroblasts, keratinocytes, dermal papilla cells, mesenchymal stem cells of bone marrow and adipose tissue, neurons and endothelial cells, primary germ cells, pluripotent stem cells. Using our experience and the experience of our colleagues, active clinicians, a set of methods was developed for a new direction of medical science - regenerative medicine.
In mice model, we showed that skin equivalent promoted immediate filling of wound bed and provided simultaneous reorganization of dermal component into highly vascularized granulation-like tissue and rapid epithelialization, thus improving the quality of healing. Inflammation was delayed and less pronounced (Chermnykh et al., 2018).
Living skin equivalents developed in our laboratory are used in treatment of burn patients and patients with chronic wounds.
Pluripotent stem cells
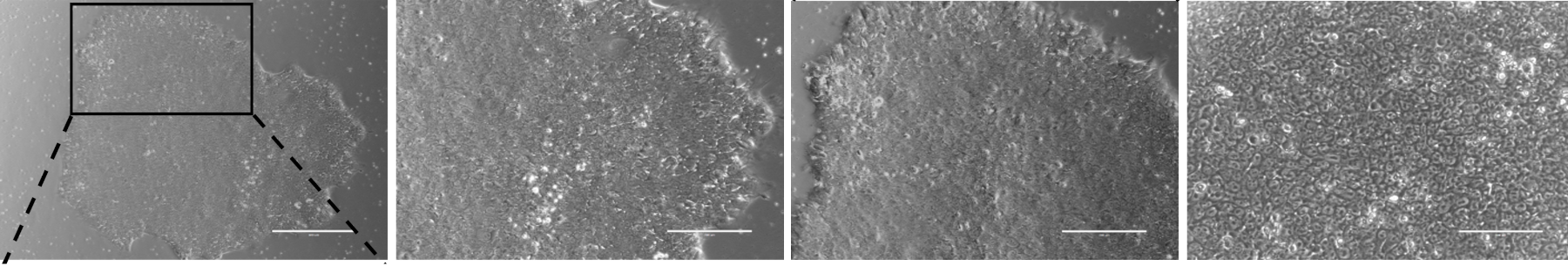
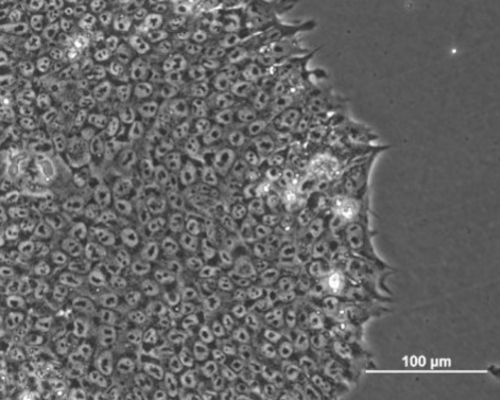
Fig. 7. Phase contrast images of human induced pluripotent stem cell (hIPSCs) colony.
Pluripotency is an acquired state of blastocyst inner cell mass of preimplantation embryo in vitro. In early embryogenesis, the limitless expansion and infinite self-renewal locked these cells into a very short spatio-temporal frameworks. Therefore, the intriguing question consists of how the infinite symmetrical expansion of pluripotent stem cells can be squeezed into a short period of embryogenesis and subsequently how to further transcriptional perturbation of pluripotency genes are managed through the development.
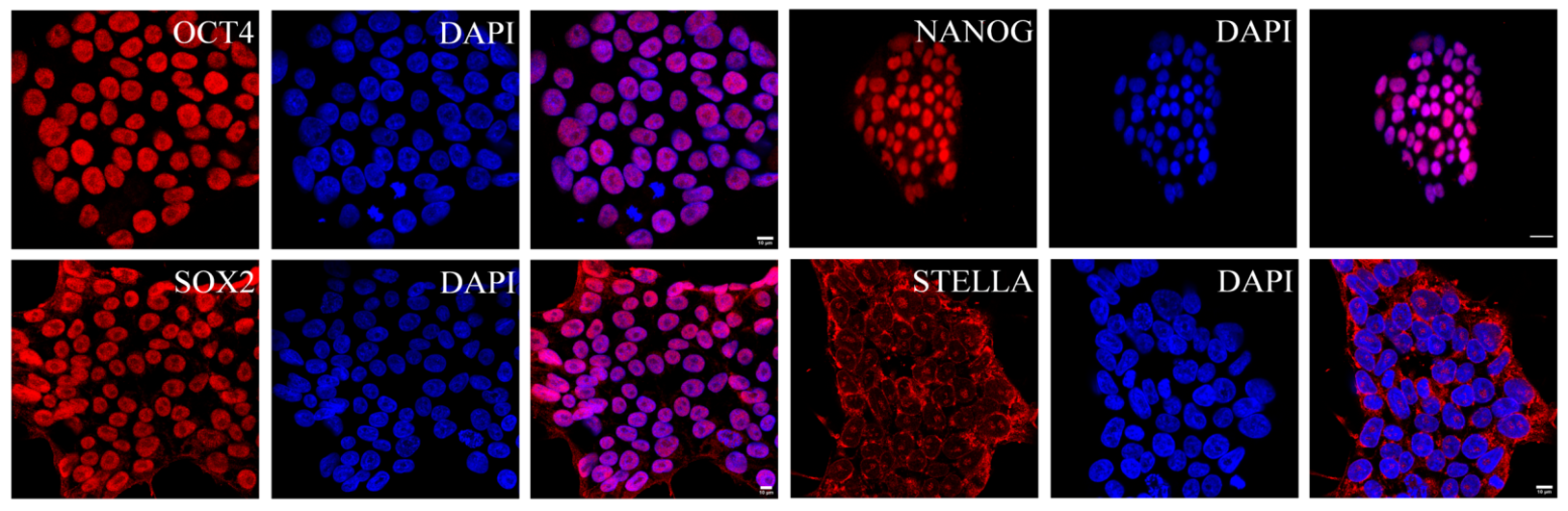
Fig. 8. Human induced pluripotent stem cells (hIPSCs) are expressing OCT4, SOX2, NANOG, STELLA (red) maintained in conventional conditions (Abdyev et al., 2021).
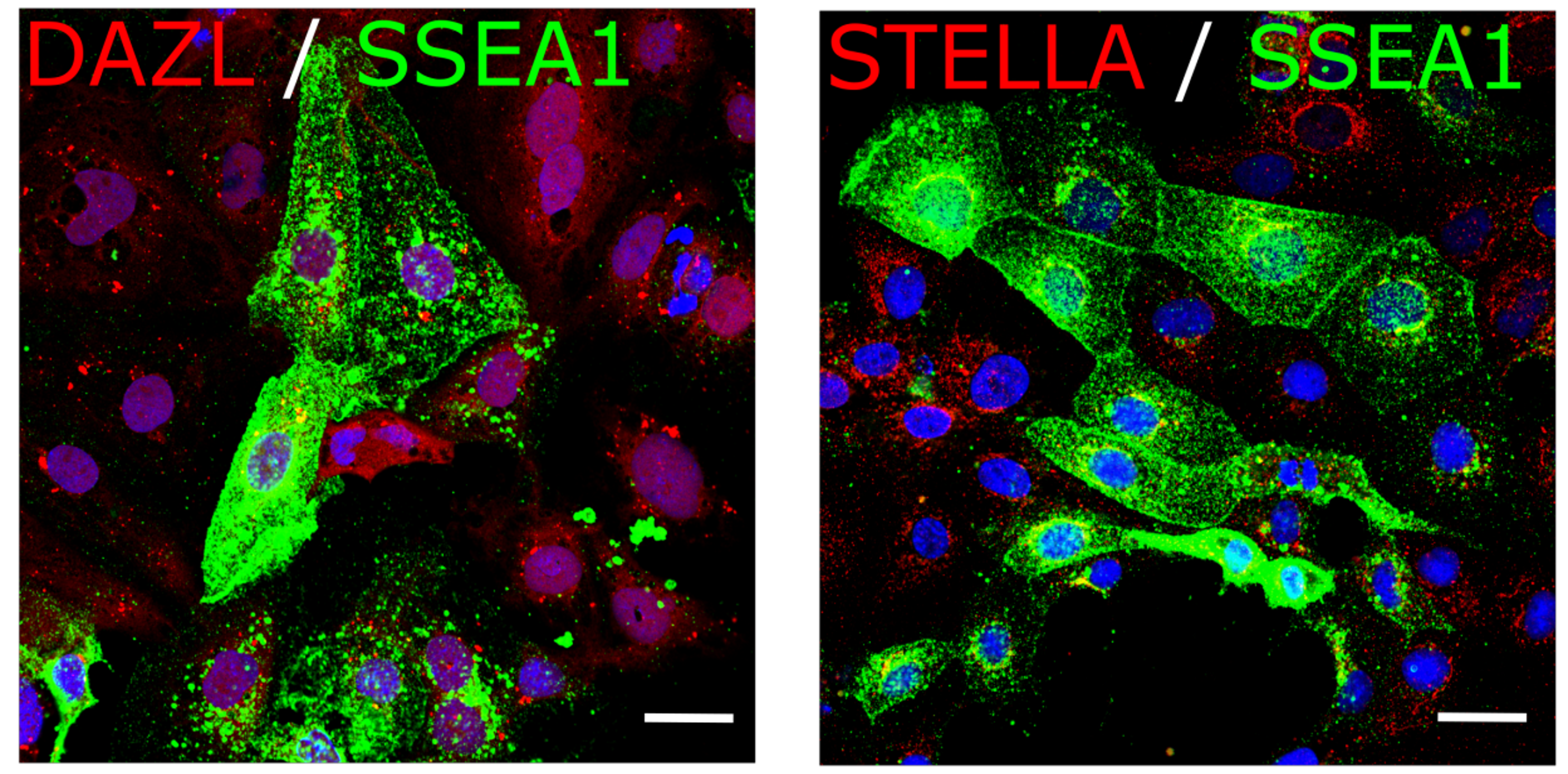
Fig. 9. hIPSCs were differentiated into hPGCLCs which express PGC markers DAZL, STELLA (red) and SSEA1 (green).
Besides addressing the above questions we derive human germ cell-like cells from pluripotent stem cells to enlighten the early gametogenesis in human development since early post-implantation embryos are not ethically and legally allowed to study. In addition, trophectoderm is the first differentiating cell type in early embryogenesis, which is an extraembryonic component. So the curiosity brought us to study the plasticity of pluripotent stem cells and attempt to derive trophectoderm.
Embryo-uterine interactions
Main topic of our study in this field is the process of embryo implantation. The implantation process is an attachment of an embryo to the uterus wall. We approach this issue by modeling embryo implantation in vitro. We are particularly interested in tissue engineering and in 3D constructs (group of models in which cells reside in 3D scaffold) as a substrate for in vitro implantation. Currently we've been developing such model that can mimic some aspects of the embryo-endometrium interaction. In our model we use epithelial and stromal cells isolated from mouse endometrium.
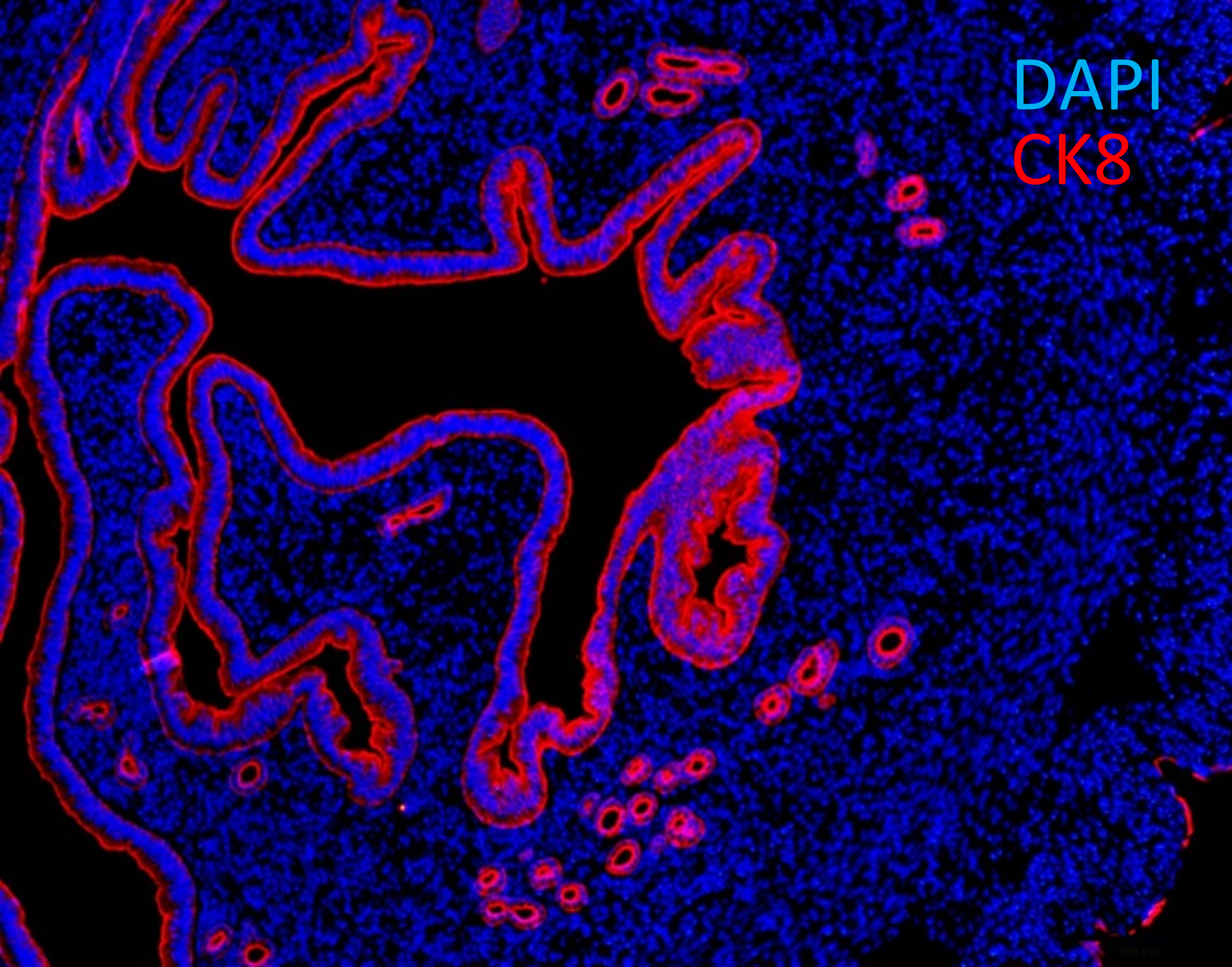
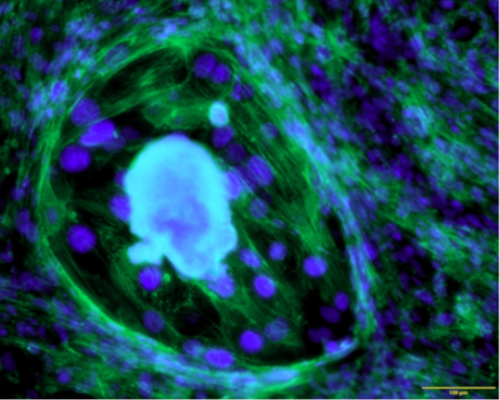
Fig. 10. Cross-section of murine uterus with endometrial epithelial cells expressing cytokeratin 8 (CK8, red).
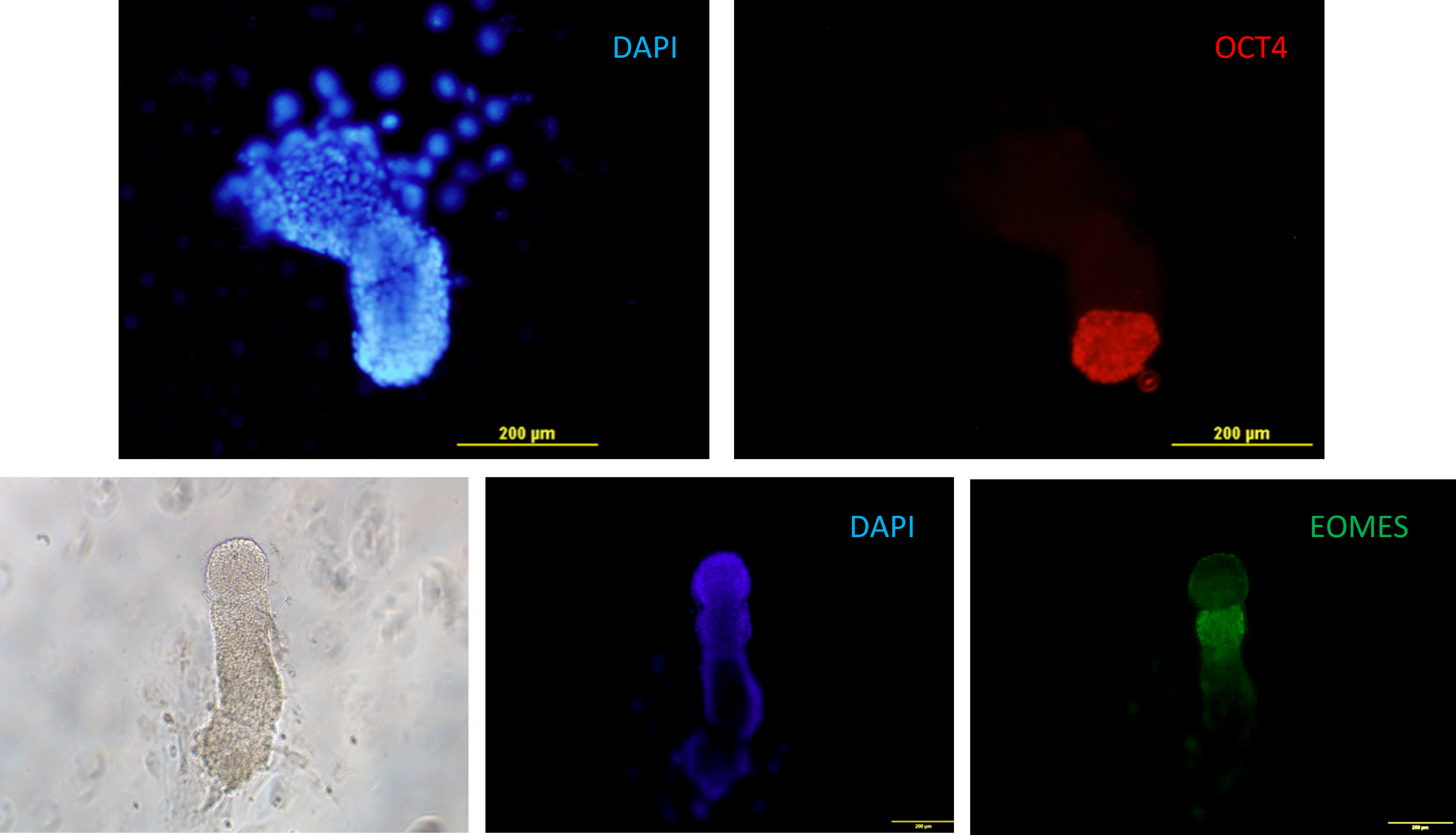
Fig. 12. Murine embryos at the stage of egg cylinder grown in vitro on plastic from blastocyst stage. Epiblast expressing Oct4 (red), extraembryonic mesoderm expressing eomesodermin (EOMES, green) .
We are also planning to study early postimplantation development of mouse embryo using our implantation model. For the implantation, response of luminal epithelium to the hormonal and embryo-derived signals is crucial. To develop 3D equivalent of mouse endometrium we are also trying to understand mechanisms of functioning of endometrial epithelial and stromal cells as well as their interactions and responses to hormones using 2D cultures of epithelial and stromal cells isolated from mouse endometrium.
And last but not least of our research topics is to locate and describe the elusive population of endometrial stem/progenitor cells. This population is responsible for cyclical regeneration of endometrium and presumably plays role in diseases such as endometriosis and endometrial cancer.
Lab members

Ekaterina Vorotelyak
Head of the Lab, Doctor of Biological Sciences, Corresponding Member of the Russian Academy of Sciences
Research interests: skin biology, hair follicle biology, cell differentiation, morphogenesis, disease modeling, tissue engineering, cell-cell interaction.

Vasily Terskikh
Chief researcher, Doctor of Bilological sciences, Professor
Research interests: regeneration of the epidermis and various epithelia of human and other animals; creation of tissue equivalents and development of methods for their transplantation for the restoration of various epidermal lesions, including deep burns and extended wound surfaces.

Andrey Vasiliev
Director of IDB RAS, PhD, Doctor of Biological Sciences, Professor, a Corresponding Member of the Russian Academy of Sciences

Olga Rogovaya
Senior researcher, PhD
Research interests: Biomedicine, Regenerative medicine, cell biology, development of biomedical cell products, stem cells, tissue regeneration.

Ekaterina Kalabusheva
Senior researcher, PhD
Research interests: skin regeneration and homeostasis, hair follicle, skin dermal and epidermal stem cells, skin development, skin cell self-organization, diabetes skin wound healing, iPSC differentiation, keratinocyte differentiation, chromatin organization, keratin expression.

Vepa Abdyyev
Researcher, PhD
Research interests: pluripotent stem cells, naïve and primed PSCs, stem cell biology, human Primordial germ cells, differentiation, trophectoderm specification and blastoid formation, human early embryogenesis.

Elina Chermnykh
Researcher, PhD
Research interests: skin cell biology, morphogenesis of hair follicle, regenerative medicine, extracellular matrix, wound healing, animal models of alopecia.

Irina Govorova
Researcher, PhD
Research interests: Lung cell differentiation and development, 3D lung organoids.

Elena Morgun
Researcher, PhD
Research interests: necroptosis, regeneration, programmed cell death, hair follicle, skin.

Yana Tatarenko
Junior researcher, PhD

Maria Chernyshova
Junior researcher, PhD

Oksana Sutyagina
Junior researcher, PhD
Research interests: intercellular interactions.

Olga Borisova
Research assistant

Yana Luchinina
Research assistant

Andrey Ryabinin
Research assistant

Anna Gaidamaka
Research assistant, PhD student
Research interests: embryo implantation, endometrial stem cells, endometrial epithelium, organoids.

Olesia Serbina
Research assistant, PhD student
Research interests: cell biology, genetics, clinical embryology, molecular-genetic mechanisms of the development of hereditary diseases, cellular mechanisms of muscular dystrophy.

Evgeniy Ruchko
Research assistant, PhD student
Research interests: Pancreatic beta cells, cell regeneration, diabetes.

Olga Cherkashina
Research assistant, PhD student
Research interests: cell biology, biomedicine, skin biology, skin regeneration, keratinocyte proliferation and differentiation.

Maria Shitova
Research assistant

Anfisa Ryabchenko
Research assistant
Research interests: cell biology, cell culture.

Maria Pankratova
Research assistant
Selected publications
- Gnedeva K., Vorotelyak E., Cimadamore F., Cattarossi G., Giusto E., Terskikh VV, Terskikh AV Derivation of hair-inducing cell from human pluripotent stem cells // PLoS One. 2015 Jan 21;10(1):e0116892. doi: 10.1371/journal.pone.0116892. eCollection 2015.
- Dashinimaev EB, Artyuhov AS, Bolshakov AP, Vorotelyak EA, Vasiliev AV. Neurons Derived from Induced Pluripotent Stem Cells of Patients with Down Syndrome Reproduce Early Stages of Alzheimer's Disease Type Pathology in vitro. J Alzheimers Dis. 2017. 56(2):835-847.
- Rippa A.L., Kalabusheva E.P., Vorotelyak E.A. Regeneration of Dermis: Scarring and Cells Involved // CELLS. 2019. V. 8. Is. 6. DOI: 10.3390/cells8060607.
- Kalabusheva E.P., Vorotelyak E.A. Generation of Hair Follicle Germs In Vitro Using Human Postnatal Skin Cells//In: Molecular Dermatology. Methods in Molecular Biology. – 2020. – Vol. 2154. – P. 153-163. DOI: 10.1007/978-1-0716-0648-3_13. – Botchkareva N., Westgate G. (eds). – Humana, New York, NY. Print ISBN 978-1-0716-0647-6. Online ISBN 978-1-0716-0648-3.
- Beilin A.K., Evtushenko N.A., Lukyanov D.K., Murashkin N.N., Ambarchian E.T., Pushkov A.A., Savostyanov K.V., Fisenko A.P., Rogovaya O.S., Vasiliev A.V.1, Vorotelyak E.A., Gurskaya N.G. Signatures of Dermal Fibroblasts from RDEB Pediatric Patients//Int. J. Mol. Sci. 2021. 2021. Vol. 22. Is. 4. Art. No 1792. DOI: 10.3390/ijms22041792. – Q1.
- Evtushenko N.A., Beilin A.K., Dashinimaev E.B., Ziganshin R.H., Kosykh A.V., Perfilov M.M., Rippa A.L., Alpeeva E.V., Vasiliev A.V., Vorotelyak E.A., Gurskaya N.G. hTERT-Driven Immortalization of RDEB Fibroblast and Keratinocyte Cell Lines Followed by Cre-Mediated Transgene Elimination//International Journal of Molecular Sciences. 2021. Vol. 22. Is. 8. Art no. 3809. DOI: 10.3390/ijms22083809. – Q1.
- Morgun E.I., Vorotelyak E.A. Epidermal Stem Cells in Hair Follicle Cycling and Skin Regeneration: A View From the Perspective of Inflammation//Front. Cell Dev. Biol. – 2020. . – Art. No 581697. DOI: 10.3389/fcell.2020.581697. – Q1.
- Abdyyev V.K., Sant D.W., Kiseleva E.V., Spangenberg V.E., Kolomiets O.L., Andrade N.S., Dashinimaev E.B., Vorotelyak E.A., Vasiliev A.V. In vitro derived female hPGCLCsare unable to complete meiosis in embryoid bodies//Experimental Cell Research. – 2020. – Art. No 112358. DOI: 10.1016/j.yexcr.2020.112358. – Q2.
Funding
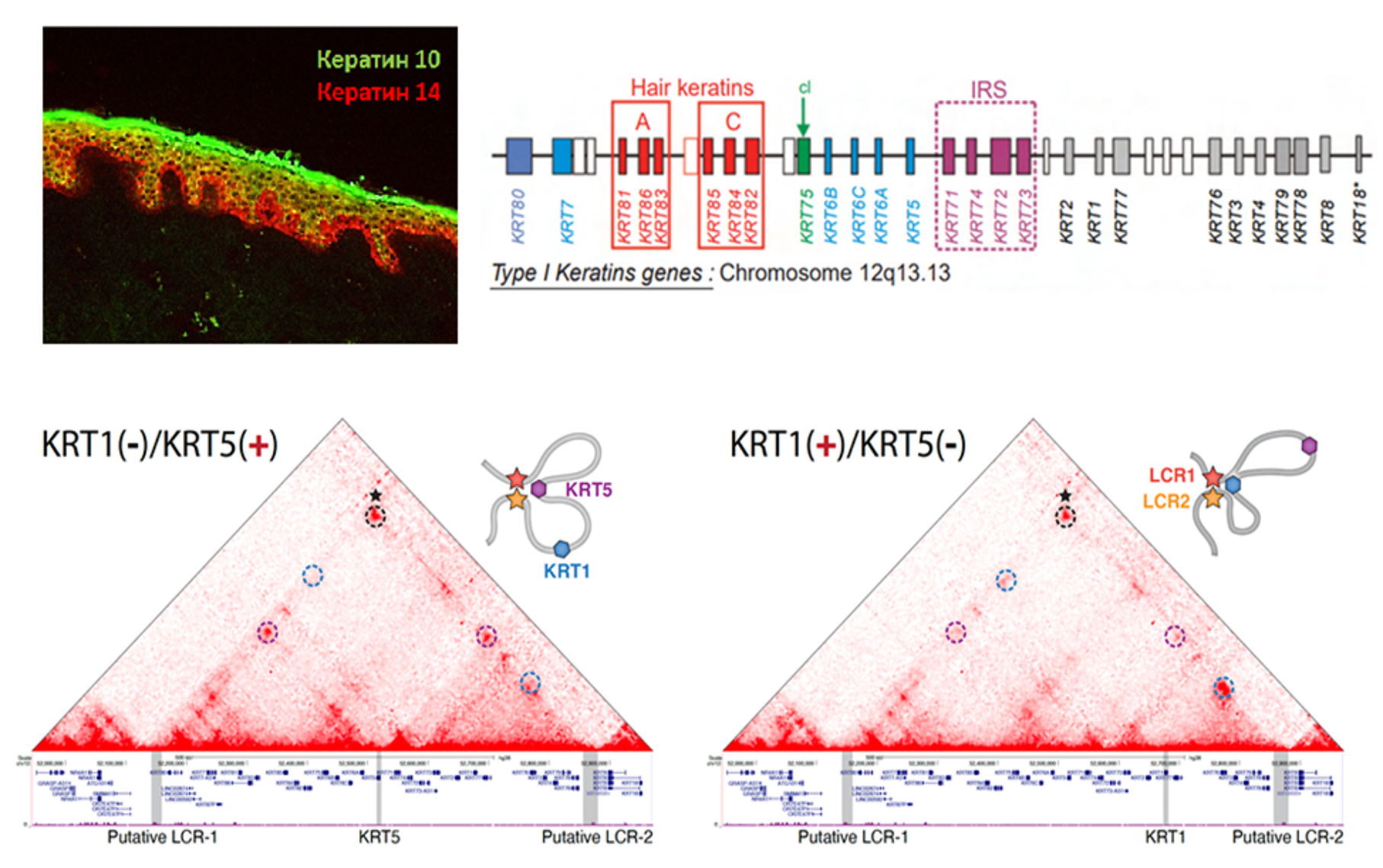
Fig. n. Upper left corner: Expression of keratin 10 (green) and keratin 14 (red) in human epidermis. Bottom of the picture: 3D conformation of keratin gene locus during skin cell differentiation.
We accepted Grant from Russian Science Foundation which aims to investigate how cells of stroma in the skin, lungs and endometrium maintain and affect epithelial cells and their specialization, how they can be used to manipulate epithelial cell fate and prevent functional degradation because of fibrosis or aging. Another objective is to find special stromal cells that can maintain epithelial stem cell identity, therefore preserving the ability of tissue to regenerate. Single cell transciptomics will be used to elucidate stromal composition of skin, lungs and endometrium at different locations and time points of development and adult life.
The project supported by Russian Ministry of Science and Education will develop genetic technological approaches for studying, modeling and search for therapy of serious diseases like Parkinson disease, lung fibrosis, mental disorders, diabetes, etc.
The project “Investigation of the mechanisms of switching keratin gene expression during epidermal differentiation” funded by Russian Fund of Basic Research is carried out in collaboration with Institute of Gene Biology Russian Academy of Sciences. A keratin gene expression profile is an integral characteristic of epithelial cells and determines their functionality, tissue and organ specificity, normal or pathological phenotype. We applied highresolution chromosome conformation capture method, CTALE (Chromatin TArget Ligation Enrichment) to build the map of spatial chromatin organization of 12q13.13 locus in the human epidermal skin keratinocytes at two distinct stages of differentiation. We found that expression switching between keratin 5 (specific for basal epidermal keratinocytes) and keratin 1 genes (actively transcribed in spinous K1/K10positive keratinocytes) is accompanied by drastic changes in chromatin loop profile inside the locus. Both genes in active state spatially interact with two regions located at 5’ and 3’flanks of the locus, and loose these contacts upon inactivation. Comparison with publicly available datasets showed that both regions identified possess the features characteristic for LCRs: high level of histone H3 acetylation at K27 position, presence of numerous DNAse I hypersensitivity sites, binding of CTCF and transcription factors involved into keratin transcription regulation. These data potentially denote that transcription switching within keratin gene domain is controlled by two locus control regions forming chromatin loops with active keratin promoters.
Collaborations
- Sanford Burnham Prebys Medical Discovery Institute (Alexey Terskikh Lab), USA
- Institute of Gene Biology (Sergey Razin Lab), Russian Academy of Sciences
- Insitute of Cytology, St.-Petersburg, Russian Academy of Sciences
- Moscow State University, Biological Faculty
- Privolzhskyi Medical Research University
- Pirogov Russian National Research Medical University
- Institut Gustave Roussy, France